Quantitative determination of indicators of the composition and properties of urine using the "dry chemistry" method (principles of the method, analytical characteristics)
Urine analyzers on test strips have long become reliable medical assistant for clinical laboratory diagnostics. Today, it is hardly possible to find a laboratory that is not equipped with devices of this type, which allow conducting a simultaneous urine test for 10–13 indicators within 1–2 minutes. The only drawback of these devices is the semiquantitative analysis result. Thus, in a large percentage of cases, to refine the analysis results, it is necessary to repeat the study using quantitative methods. The employees of Eiliton LLC asked themselves whether it is possible to create a device that works according to the “dry chemistry” method and which allows one to obtain quantitative analysis results. The task was solved. This article presents the results of a study of the characteristics of the analytical system "urine analyzer URiSKAN-strip (LLC Eiliton, Russia) + test strips Uriscan 11 strip (YD Diagnostics, South Korea)." Using the example of the “Protein” test zone, it is shown that this analytical system allows you to obtain results that accurately meet the requirements for quantitative methods for measuring protein concentration in urine. The use of the URiSCAN-strip analyzer in laboratory practice will significantly increase the accuracy and reproducibility of urine tests, while preserving the simplicity and convenience of work that is characteristic of all express urine analyzers.
Introduction Clinical analysis of urine includes the determination of a number of indicators of the molecular composition and physico-chemical properties of urine, as well as analysis of urinary sediment. The indicators of the first group include the concentration of protein (albumin), glucose, ketones, nitrites, bilirubin, urobilinogen, pH, specific gravity. They are determined mainly by the methods of "dry chemistry". Analysis of urine sediment includes the determination of red blood cells, white blood cells, epithelial cells, various types of cylinders, organic and inorganic crystals and microorganisms. As a rule, it is carried out by microscopy methods. At the same time, to determine some elements of the urinary sediment (erythrocytes and leukocytes), the methods of "dry chemistry" are also widely used. The method of “dry chemistry” is based on color reactions leading to a change in the color of the test zone of the strip. Depending on the chemical properties of the indicator being determined, these are either ordinary chemical or enzymatic reactions, and in some cases, the analyte’s own enzymatic activity is determined [1]. For example, to determine the concentration of hemoglobin (red blood cells), a chemical color reaction based on the peroxidase activity of hemoglobin is used. White blood cells are detected in the urine by registering leukocyte esterase. The color change of the test zones is determined either visually: the color of the test zone is compared with the color scale on the pencil case or using a specialized reflective photometer - urine analyzer. The latter method of evaluating the reaction is more preferable because it allows you to get an objective analysis result. A visual assessment of the reactions substantially depends both on the nature of the lighting in the room, and on the characteristics of the color perception of the laboratory assistant. In addition, the simultaneous determination of 10–13 indicators in a large number of samples is laborious. Urine analyzers used in modern laboratories based on reflective photometers are of two types. The first type of instrument implements a method for assessing the color change of test zones, similar to the color perception of the human eye. Test areas are illuminated with white light containing all wavelengths of the visible range of the spectrum. The color change of the test zone as a result of the reaction with analytes is carried out by comparing the signals from three types of selective photodetectors in three wavelength ranges: red, green and blue (RGB) (Fig. 1). The color vision of a person is similarly arranged.
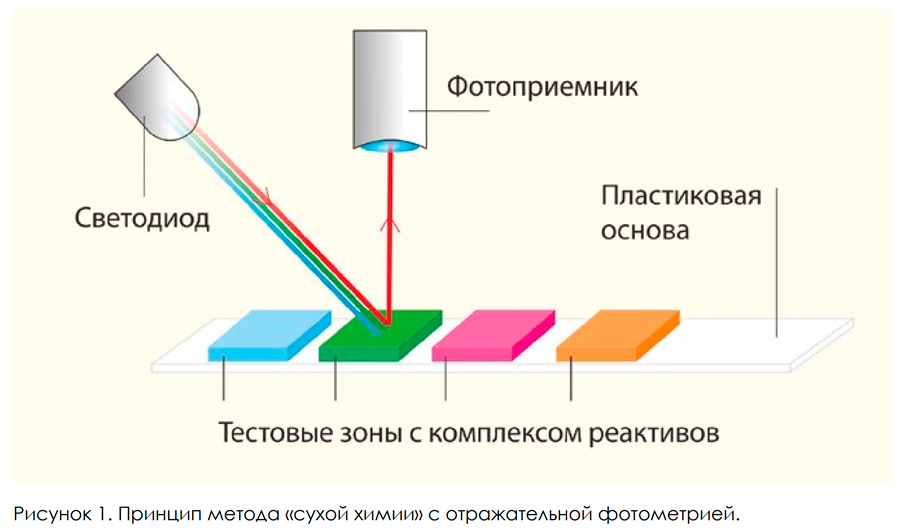
The vast majority of urine analyzers are based on this principle, including such well-known instruments in our country as URiSCAN-Optima, URiSCAN-Pro (manufactured by YD Diagnostics, South Korea), URiSCAN-pro (manufactured by Eiliton LLC, Russia). In the second type of analyzers, the method of measuring the reflection coefficient of test zones at certain wavelengths is used. For each test zone, the optimal wavelength of light is selected at which the change in the reflection coefficient has the most pronounced dependence on the concentration of analyte in the urine. In such analyzers, test zones are illuminated using LEDs with a narrow emission spectrum, and the reflected signal is detected by a non-selective photodetector (Fig. 2).
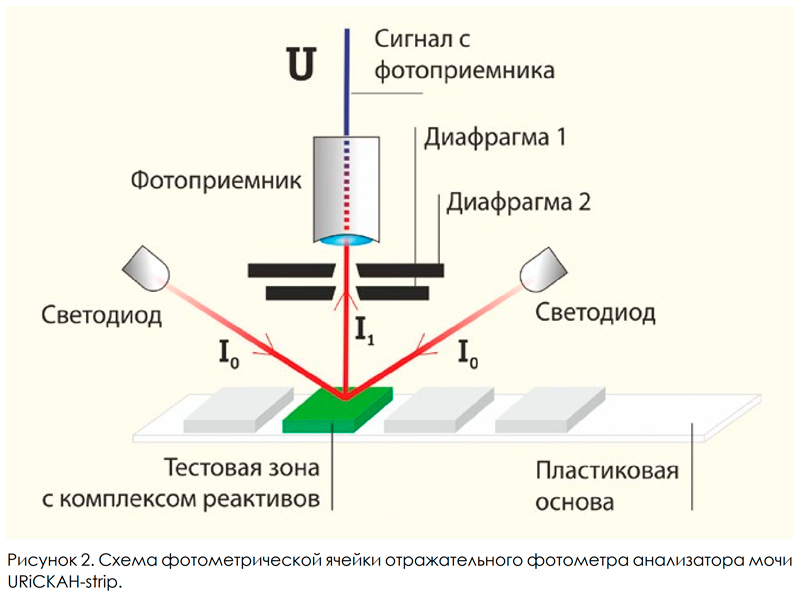
This principle is implemented in the urine analyzer URiСКАН-strip (LLC Eiliton, Russia). Urinalysis using classical methods of "dry chemistry" is performed at a semi-quantitative level. The semiquantitative result of the analysis is not a specific number (with a certain error), but a range of values in which the measured value is located. As an example, the ranges of values for semi-quantitative results of determining the concentration of protein in the urine in the table. 1 shows the concentration ranges for the analytical system "urine analyzer URiСКАН-про + test strips Uriscan 11 strip» [5].
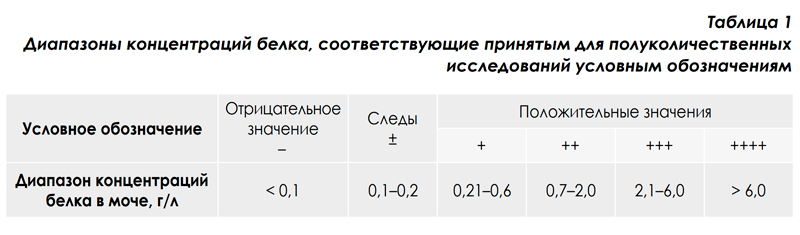
Semi-quantitative methods for urine analysis adequately solve the screening task [3, 6]. However, they are of little use for monitoring the course of the disease, including during treatment, because, as can be seen from the table. 1, even a 2-3-fold change in protein concentration during dynamic observation of the patient during the study with test strips may not be detected [3]. Therefore, for the purpose of monitoring the course of the disease and evaluating the effectiveness of treatment, improving accuracy and ensuring high reproducibility of the analysis results, which will reliably assess the dynamics of the pathological process, are extremely relevant.
Purpose of work In order to solve the problem of increasing the accuracy and reproducibility of studies, Eiliton specialists constructed a unique urine analyzer URiSCAN-strip, which allows using uriscan 11 strip test strips to obtain urine analysis results that meet the requirements of quantitative research methods. To identify and minimize the total error of the study, an analysis of the measurement errors was performed using the analytical system: URiSCAN-strip + test strips Uriscan 11 strip. During this work, it was necessary to find a connection between the measurement results and the concentration of analytes, as well as quantify the errors at each stage. Analysis of measurement errors of the urine analyzer In the analytical system: urine analyzer URiSCAN-strip + test strips Uriscan 11 strip (Fig. 2), the signal from the photodetector U is proportional to the intensity of the reflected light incident on it from the analytical zone I1, so U = FI1. In turn, the intensity of the reflected light I1 is proportional to the intensity of the light incident on the analytical zone from the LEDs I0 and the reflection coefficient: I1 = I0 (t) Kotr (t, C). The reflection coefficient varies with time, which is due to the reactions taking place in the analytical zone, and depends on the analyte concentration ©. In the end, we get the so-called measuring function of the analytical system: U = FI0 (t) Kotr (t, C). The variations of the measured signal δU are related to the variations of the components of the analytical system as follows:
δU = (δF) I0 Котр + (δI0 ) F Котр + (δКотр) F I0
Here: δF - variation of the conversion coefficient of the light flux into an electric signal of the photodetector; δI0 — light intensity variations from LEDs; δKotr - variation of the reflection coefficient of the analytical zones of the test strip. The first two parameters are the characteristics of the analyzer, and the last characterizes the quality of the test strips. To reduce the variations (δF) and (δI0) in the URiSCAN-strip analyzer created by the specialists of Eiliton LLC, a special design of the photometric cell was used, shown in Fig. 2. The principle of operation of the URiSCAN-strip urine analyzer reflective photometer is as follows: the light incident on the test area (I0) from two identical LEDs diffusely reflects (I1) and passes through the diaphragm system and enters the photodetector, the signal from which (U) is detected using the analyzer electronic unit. Using a pair of LEDs with the same intensity and radiation spectrum for each test zone, as well as the diaphragm system, minimizes the effects of (δI0) and (δF). Thus, variations in the reflection coefficient of the analytical zones of the test strip (δKotr) make the main contribution to the analyte measurement error.
Study of the analytical characteristics of measuring protein concentration in urine
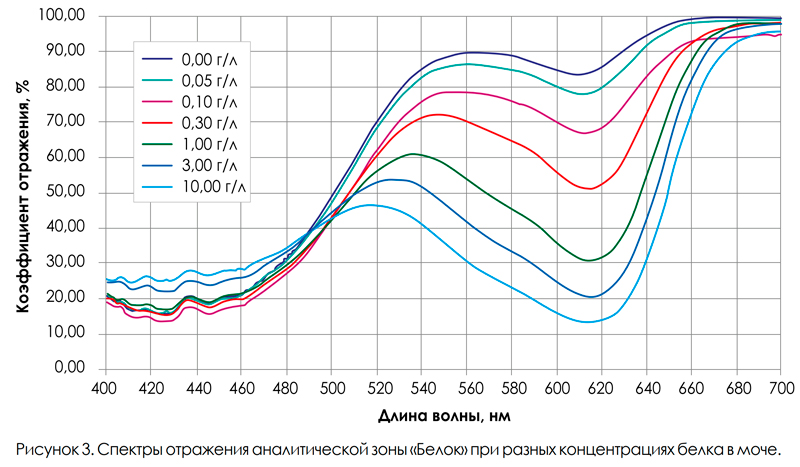
In order to determine the most optimal wavelength of LED radiation for the Belok test zone, the reflection spectra of the test strips were recorded in the entire visible wavelength range for different analyte concentrations (Fig. 3). Changes in the reflection coefficient of the Belok analytical zone are due to the formation of the protein – bromphenol blue complex in the zone. It can be seen from the spectra that, at different concentrations of protein in the urine, the most significant changes in the reflection coefficient are observed in the range of 600–630 nm. Therefore, the URiSCAN-strip analyzer uses the LEDs with a wavelength from the specified spectral range, namely 605 nm, for the Belok analytical zone. At the same time, when determining protein in urine using the methods of “dry chemistry”, one should take into account the fact that bromphenol blue is highly sensitive to the albumin fraction and much less sensitive to other protein fractions, which significantly limits the use of test strips for the diagnosis of proteinuria due to globulin fractions, mucoproteins, low molecular weight proteins, as well as Bens-Jones proteinuria [2, 3, 7].
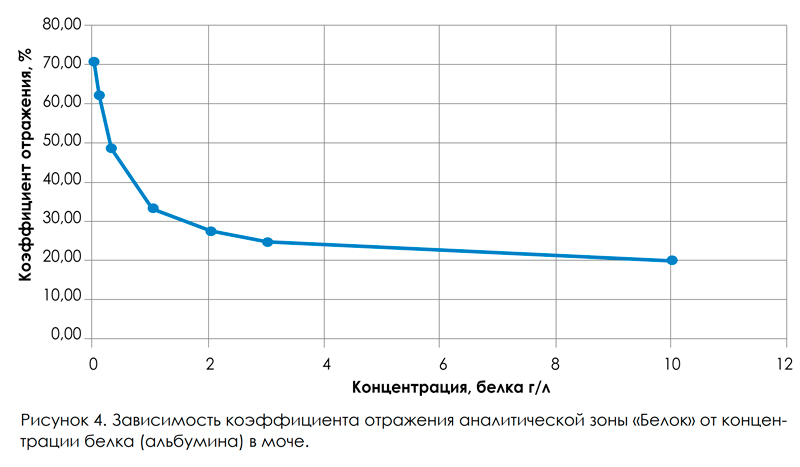
For this, urine-certified urine samples with different concentrations were prepared and a series of measurements were performed for each concentration value on the URiSCAN-strip urine analyzer. As can be seen from fig. 4, the obtained calibration curve has a nonlinear character, which is quite typical for reflective photometry. The calibration dependence of Kotr © is recorded in the memory of the URiSCAN-strip devices and is used to calculate the result of measuring the protein concentration in the studied urine samples. At the next stage, the resulting error in the determination of protein in the urine using the URiSCAN-strip analyzer was evaluated. The resulting measurement error is affected by photometer and measurement variations.
3. Reproducibility of reflection coefficient measurements
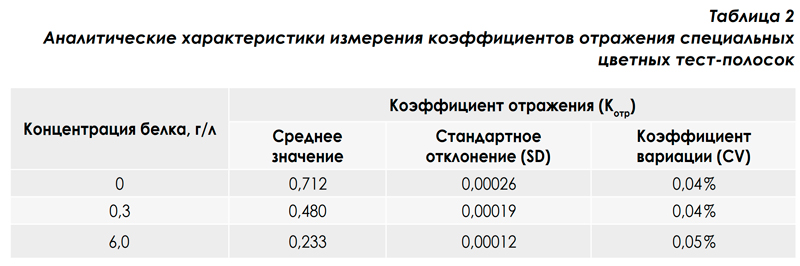
To study the variations in measuring the reflection coefficient of the photometer, special standard colored test strips were made that imitate the real urican uriscan 11 strip in their spectral characteristics. The use of standard test strips for research allows eliminating chemical variations associated with the quality and quantity of reagents in the test zone of the strip, the volume of urine applied to the test zone, the interferents contained in the urine samples, the analyte concentration, and incubation time, and evaluate exclusively photometric variations. Variations of reflection coefficients for 20 measurements in the Belok channel are presented in Table. 2. Such small variations in the measurement of reflection coefficients on the URiSCAN-strip analyzer are due to the measurement algorithm described above and the specially developed photometer design, which made it possible to eliminate variations in the entire measuring system of the device: δF and δI0 .
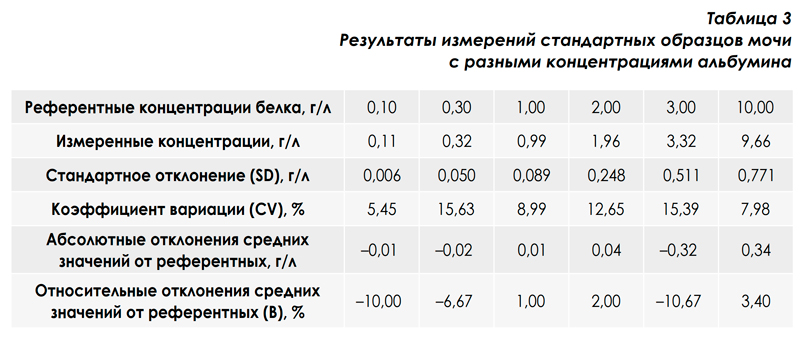
To study the reproducibility in the series and the correctness of the results of measurements of protein in the urine, standard urine samples with certified albumin concentrations were prepared. For this, several urine samples of healthy people were taken with a protein concentration close to zero. Then, strictly specified amounts of albumin were added to the pooled urine pool. The error in the preparation of standard samples did not exceed 1%. Each standard urine sample was measured 20 times and the average values of SD and CV were calculated. The measurement results are presented in table. 3. The upper line shows the certified albumin concentration values, the line below shows the average values for 20 measurements, the lower lines show the absolute and relative deviations of the average values from the certified ones. The table shows that the obtained relative deviations of the average values from the reference (B) do not exceed 10.67%, which is almost two times lower than the requirements of the order of the Ministry of Health of the Russian Federation No. 45 dated February 7, 2002 "On the system of measures to improve the quality of clinical laboratory research in health facilities Of the Russian Federation ”according to the maximum permissible values of bias (B) for a series of 20 measurements, namely: ± B20 = 20% [4]. Thus, the results obtained using the analytical system: urine analyzer URiScan-strip + test strips Uriscan 11 strip fully meet the requirements for the correct determination of protein concentration in urine by quantitative methods.
Total CV% protein measurement = (CV% photometer2 + CV% measurement2) 1/2 = (0.052 +15.632) 1/2 = 15.63%,which is noticeably lower than the maximum permissible coefficient of the total analytical variation of the protein, calculated according to the results of 20 measurements for the quantitative analysis of urine according to the order of the Ministry of Health of the Russian Federation N45 dated 7.02.2000. In accordance with this order, the maximum permissible CV20 for protein in urine is 25% [4]. It is evident that the photometer itself introduces a negligible error into the analytical system. If we consider the task of ensuring the accuracy of measurements of protein in urine wider, then it is also necessary to take into account the inter-slot variation of test strips and inter-instrument variation.
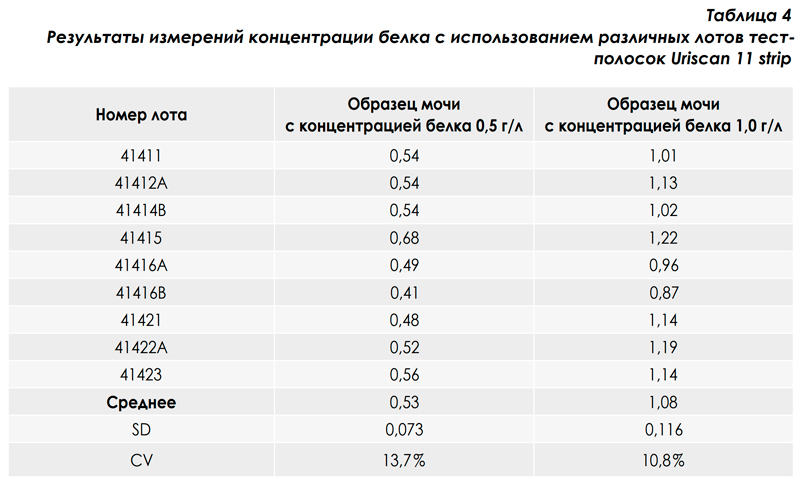
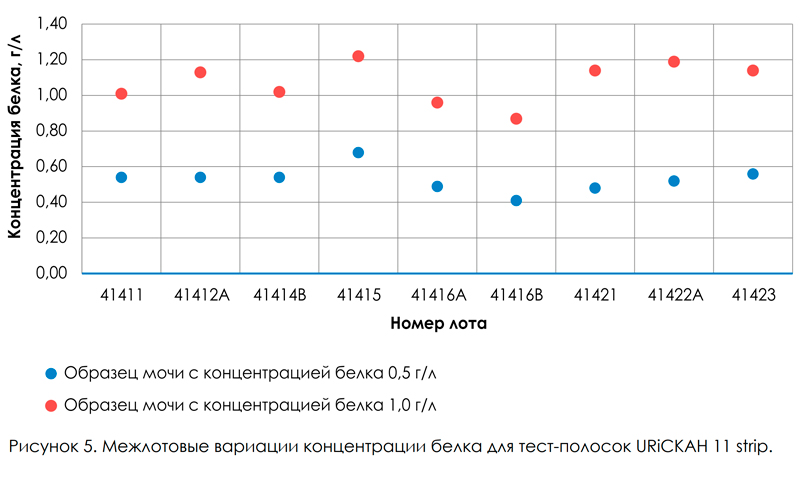
The variation due to differences in the characteristics of the test strips of different lots (series) is an important characteristic of the analytical system: urine analyzer + test strips. These differences, as a rule, are caused by the quality and quantity of reagents applied to the test zone of the strip, and are an integral characteristic of the quality of test strips of a certain brand. In the table. 4 and in fig. 5 presents data on the study of inter-lot variation in the results of measurements of protein in urine using the analytical system: URiSCAN-strip + Uriscan 11 strip test strips. From the above data it is seen that the values of inter-lot variation are close to the values of variation in the analytical series (intra-lot variation) and do not exceed 13.7%. The high comparability of the results in the determination of protein in urine using the URiSCANstrip analyzer using Uriscan 11 strip test strips of various lots indicates the consistently high quality of Uriscan 11 strip test strips.

Since the calibration of the device is entered into the memory of each device during its production and does not imply a calibration procedure in laboratories, an important characteristic of such devices is the inter-instrument reproducibility. To study this characteristic, 40 instruments were taken, and one measurement was performed on each of them. The results are presented in table. 5. Comparing accuracy indicators in table. 3, 4 and 5, we can conclude: the inter-instrument variation is less compared to the variation in the analytical series and is comparable to the inter-lot variation. The results obtained indicate that when performing urinalysis in different laboratories equipped with URiSCAN-strip analyzers, comparable results will be obtained and it is not necessary for the patient to monitor urine in the same laboratory when monitoring the pathological process in dynamics. So, having obtained the data of inter-slot and inter-instrument variation, we can estimate the total error in the determination of protein in urine in the range of 0.1-10.0 g / l under the most extreme conditions:
CV%protein measurements = (CV% photometer 2 + CV% measurements 2 + CV% inter-slot 2 + CV% inter-instrument 2) 1/2 = (0.052 + 15.63 + 13.72 + 13.32) 1/2 = 24.7%Thus, even taking into account all possible types of variations, the total protein determination error does not exceed 25%!
Findings
The results presented in this paper showed that the analytical system: URiSCAN-strip + test strips Uriscan 11 strip has the following characteristics:- Variations in the measurement of reflection coefficients do not exceed 0.05%, which is a unique characteristic for devices of this class, which allows for high accuracy of the study of urine parameters on the URiSCAN-strip analyzer;
- the relative deviations of the average values from the reference (B) do not exceed —10.67%, which fully complies with the requirements of the order of the Ministry of Health of the Russian Federation of 07.02.2000 N 45 “On the system of measures to improve the quality of clinical laboratory studies in healthcare institutions of the Russian Federation” to the correct determination of concentration protein in urine by quantitative methods;
- the coefficient of variation in an analytical series of 20 measurements in the range of protein concentrations in the urine of 0.1-10.0 g / l does not exceed 15.63% and fully meets the requirements of the order of the Ministry of Health of the Russian Federation of 07.02.2000 N 45 for quantitative analysis of urine;
- the values of inter-lot variation are close to the values of variation in the analytical series (intra-lot variation) and do not exceed 13.7%, which indicates the stable quality of the Uriscan 11 strip test strips;
- the inter-instrument variation is smaller compared to the variation in the analytical series. The coefficient of variation of the results of determination of protein concentration on 40 instruments varies in the range of 9.9–13.3%, which ensures comparability of the results of protein studies in urine in different laboratories in the region equipped with URiSCAN-strip analyzers. The analytical characteristics of the presented system, which are so high for the methods of “dry chemistry”, were achieved due to the use by Russian scientists of unique design and software solutions when creating the URiSCAN-strip urine analyzer and the use of high-quality Uriscan 11 strip test strips.
Bibliography
- Volkova I.A. General urine analysis at the present stage of development of clinical laboratory diagnostics. // Laboratory of MPI.— 2014.— Special issue No. 5.— P. 59–63.
- Ivanova V.N., Pervushin Yu.V., Rogova S.Sh., Abasova T.V. Interpretation of urine test results. // www.stgmu.ru/userfiles/depts/clinical_lab_diagnosis_ pe/Obschij_analiz_mochi.rtf.
- Kurilyak O.A., Shibanov A.N. Urinalysis in the laboratory of a modern clinic. // Clinic. Special issue No. 12, 2018. “Laboratory of MPI” P. 24-30.
- Order of the Ministry of Health of the Russian Federation No. 45 of 02/07/2000 // Quality management of clinical laboratory research. Regulations. Moscow. - Labpress, 2000, S. 5–57.
- URiSCAN-pro urine analyzer instruction manual. Moscow, 2017, p. 34.
- Shibanov A.N., Kurilyak O.A. Laboratory - to the clinician. Urinalysis in a modern clinic. // Medical alphabet. - 2017. - Volume 3 (Hospital - all for healthcare facilities). No. 33. S. 54-60.
- Emanuel V.L. Laboratory technology for the assessment of urinary syndrome. // Nephrology.— 2007, Volume 11, No. 1.— P. 17–27.
A.N. Shibanov, Ph.D. n .; O.A. Kurilyak, K. b. n .; A.V. Tsyganova; P.A. Starikov; I. M. Yelkina